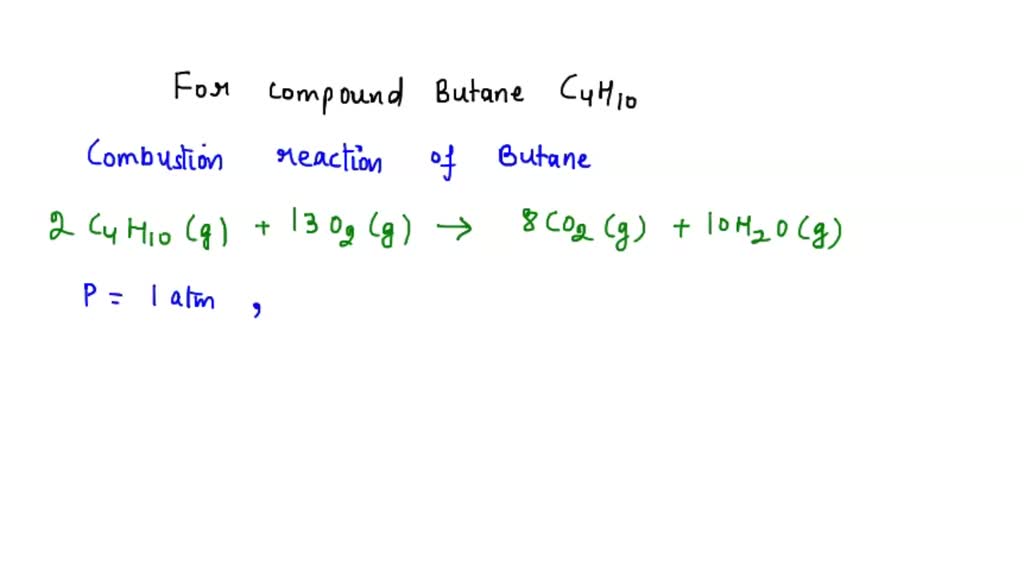
Butane Burning In A Lighter Is An Example Of A Chemical Change . The act of burning paper actually results in the formation of. when oxygen is plentiful, butane burns to form carbon dioxide and water vapor as observed in modern lighters (figure. one good example of a chemical change is burning a candle. (a) butane burning in a butane lighter. When butane burns, the following change takes place. How many grams of o2 are needed to. which changes involve a chemical reaction? the fuel in a lighter is butane. the balanced chemical equation for the combustion of butane (c4h10) is given below. Study with quizlet and memorize flashcards containing terms like natural gas burns in stove, liquid propane in a gas grill. in a chemical change, the starting and ending materials have a different chemical composition. 2c4h10 + 13o2 → 8co2 + 10h2o is this a. when oxygen is plentiful, butane burns to form carbon dioxide and water vapor as observed in modern lighters (figure \(\pageindex{1}\)).
from www.numerade.com
which changes involve a chemical reaction? When butane burns, the following change takes place. one good example of a chemical change is burning a candle. when oxygen is plentiful, butane burns to form carbon dioxide and water vapor as observed in modern lighters (figure \(\pageindex{1}\)). (a) butane burning in a butane lighter. the balanced chemical equation for the combustion of butane (c4h10) is given below. The act of burning paper actually results in the formation of. in a chemical change, the starting and ending materials have a different chemical composition. 2c4h10 + 13o2 → 8co2 + 10h2o is this a. How many grams of o2 are needed to.
SOLVED Butane, C4H10, is a component of natural gas that is used as fuel for cigarette lighters
Butane Burning In A Lighter Is An Example Of A Chemical Change which changes involve a chemical reaction? (a) butane burning in a butane lighter. when oxygen is plentiful, butane burns to form carbon dioxide and water vapor as observed in modern lighters (figure \(\pageindex{1}\)). Study with quizlet and memorize flashcards containing terms like natural gas burns in stove, liquid propane in a gas grill. which changes involve a chemical reaction? How many grams of o2 are needed to. When butane burns, the following change takes place. 2c4h10 + 13o2 → 8co2 + 10h2o is this a. The act of burning paper actually results in the formation of. in a chemical change, the starting and ending materials have a different chemical composition. one good example of a chemical change is burning a candle. the balanced chemical equation for the combustion of butane (c4h10) is given below. when oxygen is plentiful, butane burns to form carbon dioxide and water vapor as observed in modern lighters (figure. the fuel in a lighter is butane.
From pdfprof.com
la combustion du gaz butane Butane Burning In A Lighter Is An Example Of A Chemical Change When butane burns, the following change takes place. (a) butane burning in a butane lighter. 2c4h10 + 13o2 → 8co2 + 10h2o is this a. the fuel in a lighter is butane. when oxygen is plentiful, butane burns to form carbon dioxide and water vapor as observed in modern lighters (figure. the balanced chemical equation for the. Butane Burning In A Lighter Is An Example Of A Chemical Change.
From mavink.com
20 Examples Of Chemical Change Butane Burning In A Lighter Is An Example Of A Chemical Change How many grams of o2 are needed to. (a) butane burning in a butane lighter. the fuel in a lighter is butane. which changes involve a chemical reaction? 2c4h10 + 13o2 → 8co2 + 10h2o is this a. Study with quizlet and memorize flashcards containing terms like natural gas burns in stove, liquid propane in a gas grill.. Butane Burning In A Lighter Is An Example Of A Chemical Change.
From www.netexplanations.com
Chemical Change Class 9, Is Matter Around Us Pure, Chemistry Butane Burning In A Lighter Is An Example Of A Chemical Change the balanced chemical equation for the combustion of butane (c4h10) is given below. The act of burning paper actually results in the formation of. (a) butane burning in a butane lighter. in a chemical change, the starting and ending materials have a different chemical composition. 2c4h10 + 13o2 → 8co2 + 10h2o is this a. when oxygen. Butane Burning In A Lighter Is An Example Of A Chemical Change.
From www.youtube.com
Balance combustion Butane 20pct excess 85pct consumed YouTube Butane Burning In A Lighter Is An Example Of A Chemical Change in a chemical change, the starting and ending materials have a different chemical composition. one good example of a chemical change is burning a candle. when oxygen is plentiful, butane burns to form carbon dioxide and water vapor as observed in modern lighters (figure \(\pageindex{1}\)). which changes involve a chemical reaction? when oxygen is plentiful,. Butane Burning In A Lighter Is An Example Of A Chemical Change.
From pdfprof.com
La combustion du butane Butane Burning In A Lighter Is An Example Of A Chemical Change Study with quizlet and memorize flashcards containing terms like natural gas burns in stove, liquid propane in a gas grill. When butane burns, the following change takes place. when oxygen is plentiful, butane burns to form carbon dioxide and water vapor as observed in modern lighters (figure. in a chemical change, the starting and ending materials have a. Butane Burning In A Lighter Is An Example Of A Chemical Change.
From www.pinterest.ca
Examples of Chemical Change and How to Recognize It Chemical changes, Chemical and physical Butane Burning In A Lighter Is An Example Of A Chemical Change How many grams of o2 are needed to. in a chemical change, the starting and ending materials have a different chemical composition. the fuel in a lighter is butane. which changes involve a chemical reaction? when oxygen is plentiful, butane burns to form carbon dioxide and water vapor as observed in modern lighters (figure \(\pageindex{1}\)). When. Butane Burning In A Lighter Is An Example Of A Chemical Change.
From propanehq.com
Can Butane Burn Without Oxygen? (CO Can Be Produced) Propane HQ Butane Burning In A Lighter Is An Example Of A Chemical Change which changes involve a chemical reaction? How many grams of o2 are needed to. (a) butane burning in a butane lighter. when oxygen is plentiful, butane burns to form carbon dioxide and water vapor as observed in modern lighters (figure. the fuel in a lighter is butane. in a chemical change, the starting and ending materials. Butane Burning In A Lighter Is An Example Of A Chemical Change.
From pdfprof.com
la combustion complète du butane Butane Burning In A Lighter Is An Example Of A Chemical Change the balanced chemical equation for the combustion of butane (c4h10) is given below. one good example of a chemical change is burning a candle. which changes involve a chemical reaction? (a) butane burning in a butane lighter. How many grams of o2 are needed to. in a chemical change, the starting and ending materials have a. Butane Burning In A Lighter Is An Example Of A Chemical Change.
From sciencing.com
What Temperatures Do Lighters Burn At? Sciencing Butane Burning In A Lighter Is An Example Of A Chemical Change 2c4h10 + 13o2 → 8co2 + 10h2o is this a. when oxygen is plentiful, butane burns to form carbon dioxide and water vapor as observed in modern lighters (figure. when oxygen is plentiful, butane burns to form carbon dioxide and water vapor as observed in modern lighters (figure \(\pageindex{1}\)). the fuel in a lighter is butane. . Butane Burning In A Lighter Is An Example Of A Chemical Change.
From www.youtube.com
Burning liquid butane YouTube Butane Burning In A Lighter Is An Example Of A Chemical Change How many grams of o2 are needed to. The act of burning paper actually results in the formation of. 2c4h10 + 13o2 → 8co2 + 10h2o is this a. Study with quizlet and memorize flashcards containing terms like natural gas burns in stove, liquid propane in a gas grill. when oxygen is plentiful, butane burns to form carbon dioxide. Butane Burning In A Lighter Is An Example Of A Chemical Change.
From www.wou.edu
Chapter 6 Quantities in Chemical Reactions Chemistry Butane Burning In A Lighter Is An Example Of A Chemical Change in a chemical change, the starting and ending materials have a different chemical composition. The act of burning paper actually results in the formation of. which changes involve a chemical reaction? one good example of a chemical change is burning a candle. when oxygen is plentiful, butane burns to form carbon dioxide and water vapor as. Butane Burning In A Lighter Is An Example Of A Chemical Change.
From cepbbbdx.blob.core.windows.net
Can You Use Stove Butane In A Lighter at Thomas Meier blog Butane Burning In A Lighter Is An Example Of A Chemical Change when oxygen is plentiful, butane burns to form carbon dioxide and water vapor as observed in modern lighters (figure \(\pageindex{1}\)). (a) butane burning in a butane lighter. which changes involve a chemical reaction? Study with quizlet and memorize flashcards containing terms like natural gas burns in stove, liquid propane in a gas grill. in a chemical change,. Butane Burning In A Lighter Is An Example Of A Chemical Change.
From www.jotscroll.com
Chemical Reactions Examples and Types Jotscroll Butane Burning In A Lighter Is An Example Of A Chemical Change How many grams of o2 are needed to. which changes involve a chemical reaction? When butane burns, the following change takes place. one good example of a chemical change is burning a candle. the balanced chemical equation for the combustion of butane (c4h10) is given below. the fuel in a lighter is butane. in a. Butane Burning In A Lighter Is An Example Of A Chemical Change.
From www.adamsgas.co.uk
Popular Uses For Butane Gas Adams Gas Butane Burning In A Lighter Is An Example Of A Chemical Change in a chemical change, the starting and ending materials have a different chemical composition. (a) butane burning in a butane lighter. the balanced chemical equation for the combustion of butane (c4h10) is given below. which changes involve a chemical reaction? How many grams of o2 are needed to. Study with quizlet and memorize flashcards containing terms like. Butane Burning In A Lighter Is An Example Of A Chemical Change.
From slideplayer.com
Chapter 3 Molecules, Compounds, and Chemical Equations ppt download Butane Burning In A Lighter Is An Example Of A Chemical Change one good example of a chemical change is burning a candle. The act of burning paper actually results in the formation of. (a) butane burning in a butane lighter. When butane burns, the following change takes place. 2c4h10 + 13o2 → 8co2 + 10h2o is this a. in a chemical change, the starting and ending materials have a. Butane Burning In A Lighter Is An Example Of A Chemical Change.
From slideplayer.com
Systems and Scale Unit Activity 5.4 Other Examples of Combustion ppt download Butane Burning In A Lighter Is An Example Of A Chemical Change one good example of a chemical change is burning a candle. when oxygen is plentiful, butane burns to form carbon dioxide and water vapor as observed in modern lighters (figure. the balanced chemical equation for the combustion of butane (c4h10) is given below. which changes involve a chemical reaction? (a) butane burning in a butane lighter.. Butane Burning In A Lighter Is An Example Of A Chemical Change.
From www.thoughtco.com
An Introduction to Combustion (Burning) Reactions Butane Burning In A Lighter Is An Example Of A Chemical Change The act of burning paper actually results in the formation of. the fuel in a lighter is butane. when oxygen is plentiful, butane burns to form carbon dioxide and water vapor as observed in modern lighters (figure \(\pageindex{1}\)). When butane burns, the following change takes place. in a chemical change, the starting and ending materials have a. Butane Burning In A Lighter Is An Example Of A Chemical Change.
From www.thoughtco.com
Examples of Physical Changes and Chemical Changes Butane Burning In A Lighter Is An Example Of A Chemical Change the fuel in a lighter is butane. (a) butane burning in a butane lighter. when oxygen is plentiful, butane burns to form carbon dioxide and water vapor as observed in modern lighters (figure. the balanced chemical equation for the combustion of butane (c4h10) is given below. The act of burning paper actually results in the formation of.. Butane Burning In A Lighter Is An Example Of A Chemical Change.